distinguish between aldehyde and ketone by chemical test Difference between aldehyde and ketone
Possible post:
Have you ever wondered about the difference between aldehydes and ketones? While they both belong to the same family of organic compounds called carbonyl compounds, they have distinct structures and properties that make them useful in different ways.
Aldehydes
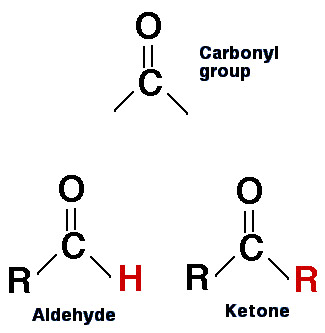
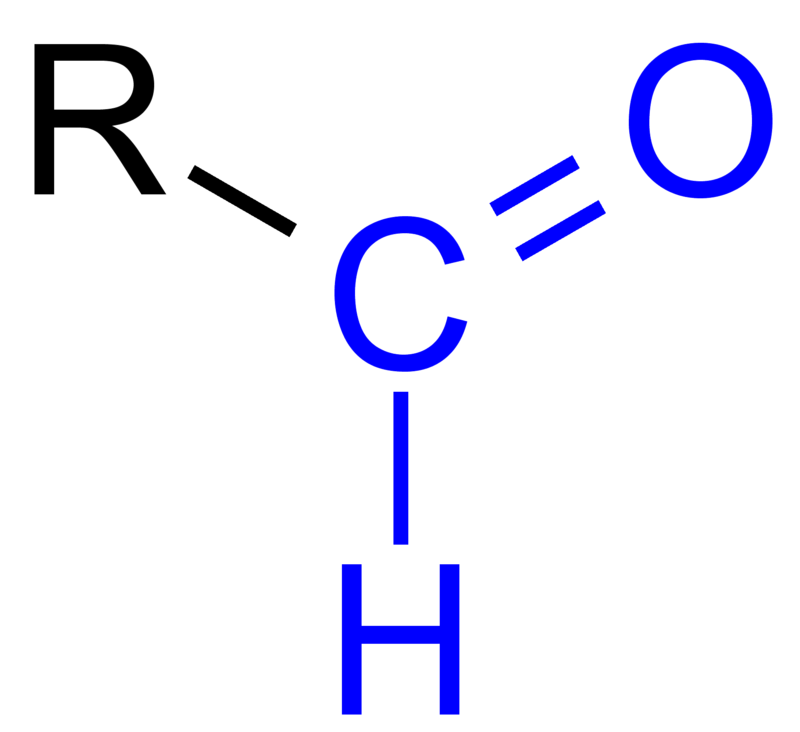
An aldehyde has a carbonyl group as its functional group, which means it is a carbon atom double-bonded to an oxygen atom, with a hydrogen atom attached on the other side. The general formula for a simple aldehyde is R-CHO, where R represents a methyl or ethyl group, for example. The name of an aldehyde usually ends with –al, such as formaldehyde, acetaldehyde, or benzaldehyde.
Aldehydes are commonly used as starting materials or reagents in organic synthesis, for example, in the preparation of alcohols, acids, and amines. They can also be found in many natural products, such as essential oils, fragrances, and flavors. For instance, cinnamaldehyde is responsible for the scent and flavor of cinnamon, while vanillin is the main component of vanilla extract.
 The aldehyde group is also important in biochemistry, as it is involved in many metabolic pathways in living organisms, such as the energy-yielding oxidation of glucose, which generates carbon dioxide and water, and the detoxification of alcohol, which converts ethanol to acetaldehyde and then to acetic acid in the liver.
The aldehyde group is also important in biochemistry, as it is involved in many metabolic pathways in living organisms, such as the energy-yielding oxidation of glucose, which generates carbon dioxide and water, and the detoxification of alcohol, which converts ethanol to acetaldehyde and then to acetic acid in the liver.
Ketones
A ketone, on the other hand, has a carbonyl group that is attached to two carbon atoms, which means it cannot be located at the end of a carbon chain like an aldehyde. The general formula for a simple ketone is R-CO-R’, where R and R’ represent alkyl or aryl groups, for example. The name of a ketone usually ends with –one, such as acetone, butanone, or cyclohexanone.
Ketones are also used as solvents or reactants in organic synthesis, especially for the production of polymers, such as nylon, and pharmaceuticals, such as steroids. They are also found in many natural products, such as carotenoids, which are responsible for the colors of fruits and vegetables, and steroids, which are hormones or precursors of hormones in animals and plants.
 The carbonyl group of a ketone can participate in various reactions, such as nucleophilic addition, oxidation, and reduction, depending on the nature and position of the substituent groups. For example, the reaction of acetone with iodine and sodium hydroxide produces iodoform, a yellow solid that smells like antiseptic and is used for disinfecting wounds.
The carbonyl group of a ketone can participate in various reactions, such as nucleophilic addition, oxidation, and reduction, depending on the nature and position of the substituent groups. For example, the reaction of acetone with iodine and sodium hydroxide produces iodoform, a yellow solid that smells like antiseptic and is used for disinfecting wounds.
Now that you know more about the difference between aldehydes and ketones, you may appreciate their importance in chemistry and biology. Remember to handle them safely and responsibly, as they can be flammable, toxic, or irritant, depending on the specific compound and conditions.
If you are looking for Ketones & aldehydes test: practice tests home you’ve came to the right web. We have 5 Pics about Ketones & aldehydes test: practice tests home like How will you distinguish between aldehyde and ketone?, Ketones & aldehydes test: practice tests home and also Ketones & aldehydes test: practice tests home. Here you go:
Ketones & Aldehydes Test: Practice Tests Home

How Will You Distinguish Between Aldehyde And Ketone?
 chemistrypage.inaldehyde ketone distinguish chemistrypage
chemistrypage.inaldehyde ketone distinguish chemistrypage
Difference Between Aldehyde And Ketone | Structure, Properties, Naming
 pediaa.comaldehyde ketone difference between aldehydes group functional formula carbon structure chemistry iv example form present
pediaa.comaldehyde ketone difference between aldehydes group functional formula carbon structure chemistry iv example form present
الفرق بين الألدهيد والكيتون - أخبار 2023
 ar.weblogographic.comAldehydes And Ketones: The Carbonyl Functional Group, Naming, Reactions
ar.weblogographic.comAldehydes And Ketones: The Carbonyl Functional Group, Naming, Reactions
 biology.reachingfordreams.comketone ketones aldehydes carbonyl group aldehyde functional structure difference between carboxylic compound biology molecule groups chemistry two acids diagram reactions
biology.reachingfordreams.comketone ketones aldehydes carbonyl group aldehyde functional structure difference between carboxylic compound biology molecule groups chemistry two acids diagram reactions
Ketones & aldehydes test: practice tests home. Aldehydes and ketones: the carbonyl functional group, naming, reactions. How will you distinguish between aldehyde and ketone?